
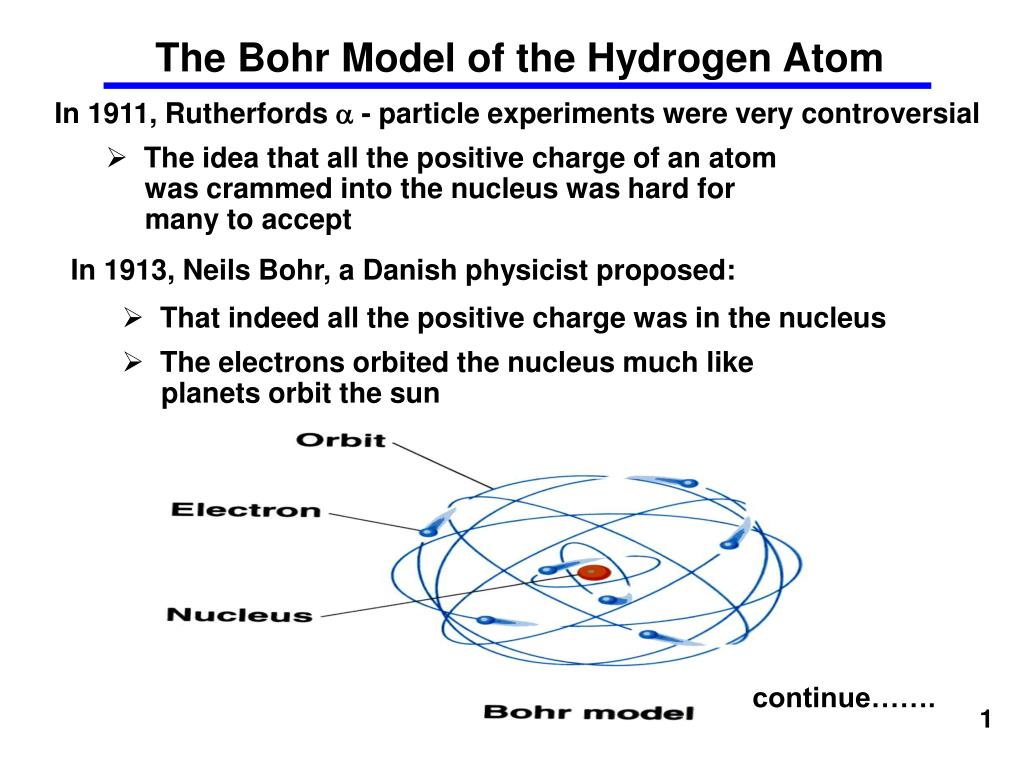
AND if they already knew that the electron was small and negative, then the atom must have a small positive nucleus with the electrons around them. Find the number of protons, electrons, and neutrons The number of protons for the atom is the same as its atomic number. This model is also called a planetary model because electrons orbiting around an atomic nucleus resemble a planet orbiting the sun. Steps to draw the Bohr Model of the atom 1. In Bohr’s atomic model, electrons are in a given orbit. The Bohr model is oversimplified and electrons do not really orbit like. Bohr’s atomic model is very close to the atomic model that can be seen these days. In the Bohr model the electron orbits the atomic nucleus (proton and neutron). If the positive alpha particles mostly passed through the foil, but some bounced back. After that, Niels Bohr presented a more refined atomic model. These orbits are called energy levels or stationary States. 2) The electrons revolve only in those orbits which have a fixed value of energy. As long as electron remains in the shell, it. 1) An atom consists of a small, heavy positively charged nucleus in the centre and electrons revolve around it in circular orbit. How could that be if the plumb pudding model was correct? Rutherford's experiment prompted a change in the atomic model. An atom has definite energy levels, and electrons revolve in those energy levels. Rutherford found that most of them went right through the foil. In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. If you shoot these positive alpha particles at this positive pudding atom, they should mostly bounce off, right? Well, that is not what happened. Bohr in order to explain why the spectrum of light from atoms was not continuous, as expected from classical electrodynamics, but had distinct spectra in frequencies that could be fitted with mathematical series, used a planetary model, imposing axiomaticaly angular momentum quantization. The correct theory of the atom is called quantum mechanics the Bohr Model is an approximation to quantum mechanics that has the virtue of being much simpler.

He shot some alpha particles (which are really just the nucleus of a helium atom) at some really thin gold foil. Bohr’s Model of an Atom In (1913,) Neil Bohr explained the stability of the atom based on the observations of his experiments.

Ernest Rutherford said one day "hey, I think I will shoot some stuff at atoms." I am sure his wife said "oh, Ernie" (she probably called him Ernie) "if it makes you happy to play with your little physics stuff, go ahead.


 0 kommentar(er)
0 kommentar(er)
